Prevalence of Csh-like fibrillar surface proteins among mitis group oral streptococci
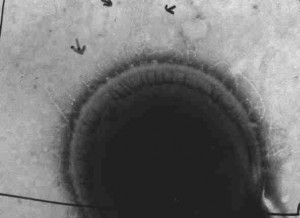
Transmission electron micrograph of Streptococcus showing cell surface structures.
The prevalence of Csh-like fibrillar surface proteins among oral streptococci was investigated by ELISA and by immunoelectron microscopy using antiserum raised to recombinant fragments of CshA of Streptococcus gordonii DL1. The majority of S. gordonii, Streptococcus sanguis and Streptococcus oralis strains tested elaborated short (ca. 50-80 nm long) surface fibrils and reacted with antiserum to the amino acid repeat region of CshA, demonstrating the widespread nature of Csh-like proteins among these species. In contrast, reactivity with antiserum raised to the adhesion-mediating non-repetitive region of CshA was more restricted. On the basis of the ELISA results, several isolates were selected for immunogold analysis using CshA antisera. Immunogold-negative staining showed a surface distribution of 10 nm gold particles consistent with antibody binding to short fibrils. Long fibrils (>150 nm long), where present, were not significantly labelled with gold. The results suggest that some of the short peritrichous fibrils on many mitis group streptococci comprise Csh-like fibrillar protein. Further, the data are consistent with our hypothesis that the antigenically conserved amino acid repeat region of Csh-like proteins forms a scaffold for cell-distal presentation of the amino-terminal non-repetitive region that, at least in S. gordonii DL1, functions as an adhesin.